What Is The Function Of Iron In The Haber Process
Iron as catalyst in the Haber Process. It provides an alternative reaction pathway with a lower activation energy.
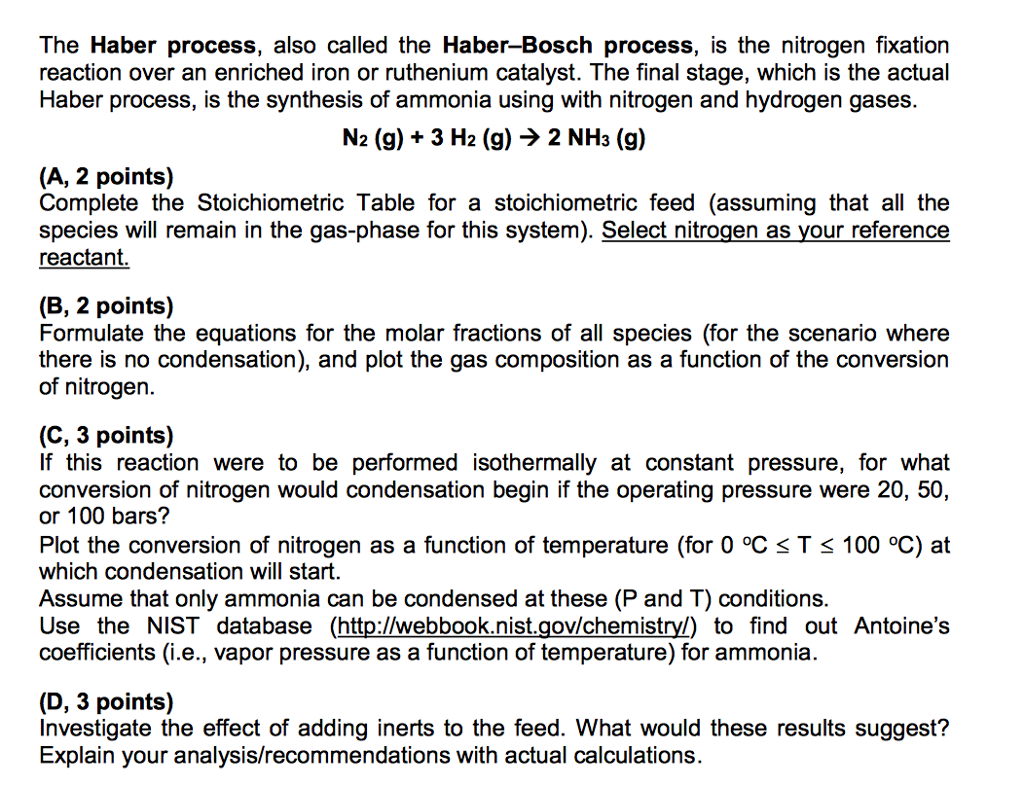 The Haber Process Also Called The Haber Bosch Pro Chegg Com
The Haber Process Also Called The Haber Bosch Pro Chegg Com
In the absence of a catalyst the reaction is so slow that virtually no reaction happens in any sensible time.

What is the function of iron in the haber process. 2020-12-31 In addition the production time of the process is shortened by using small pellets of iron to act as a catalyst. The Haber process is important because ammonia is difficult to produce on an industrial scale. 2021-01-18 Catalyst Used in Haber Process.
The Haber-Bosch process uses a catalyst or container made of iron or ruthenium with an inside temperature of over 800 F 426 C and a pressure of around 200 atmospheres to force nitrogen and hydrogen together Rae-Dupree 2011. Hydrogen is obtained by reacting natural gas. The catalyst ensures that the reaction is fast enough for a dynamic equilibrium to be set up within the very short time that the gases are actually in the reactor.
The reaction is reversible and the production of ammonia is exothermic. IFinely divided iron increases the rate of reaction. The Haber Process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia.
The raw materials for the process of making ammonia are hydrogen and nitrogen. It shifts the position of equilibrium towards the products. A brief summary of the Haber Process.
What is the function of iron in the Haber process. A flow scheme for the Haber Process looks like this. The catalyst speeds up the reaction rates enabling equilibrium to be attained in a shorter time.
The Table above sums up the. 2020-08-15 Its only function is to speed up the reaction. Iron is used as a catalyst.
2019-04-10 The process must use high pressure because nitrogen molecules are held together with strong triple bonds. HttpscuttlyFytNGyZFor more questions. He studied in many.
In the Haber process. For this question visit. IiMolybdenum acts as a promoter to increase the efficiency of the catalyst.
The Haber process also known as HaberBosch process is the reaction of nitrogen and hydrogen over an iron-substrate to produce ammonia. It reduces the enthalpy change of the reaction. Nitrogen extracted from the air and hydrogen obtained from natural gas are pumped through pipes a compressor increases the gas pressure to 200 atmospheres the pressurised.
History about the developer of Haber Process Previously known as Haber-Bosch Process was founded by Fritz Haber and Carl Bosch both who were German ChemistsFritz Haber was born in 1868 from a German-Jewish family. Iron can be used as a catalyst but the catalyst used in the production is not pure iron. The Haber Bosch Process or Haber Process is probably the most important chemical reaction in the world today.
Some notes on the conditions. The catalyst is actually slightly more complicated. You can find a full discussion about the Haber Process by following this link.
By signing up you039ll get thousands of step-by-step solutions to your homework. The Haber Process combines nitrogen and hydrogen into ammonia. It decreases the rate of the reaction.
Even though 781 of the air we breathe is nitrogen the gas is relatively inert due to the strength of the triple bond that keeps the molecule together. What is the function of iron in the Haber process. The nitrogen comes from the air and the hydrogen is obtained mainly from natural gas methane.
Usually this process takes place under high temperature and pressure. It contains potassium hydroxide as a promoter added to it to increase its efficiency. The process fixes nitrogen using nitrogen gas and hydrogen gas over an enriched iron.
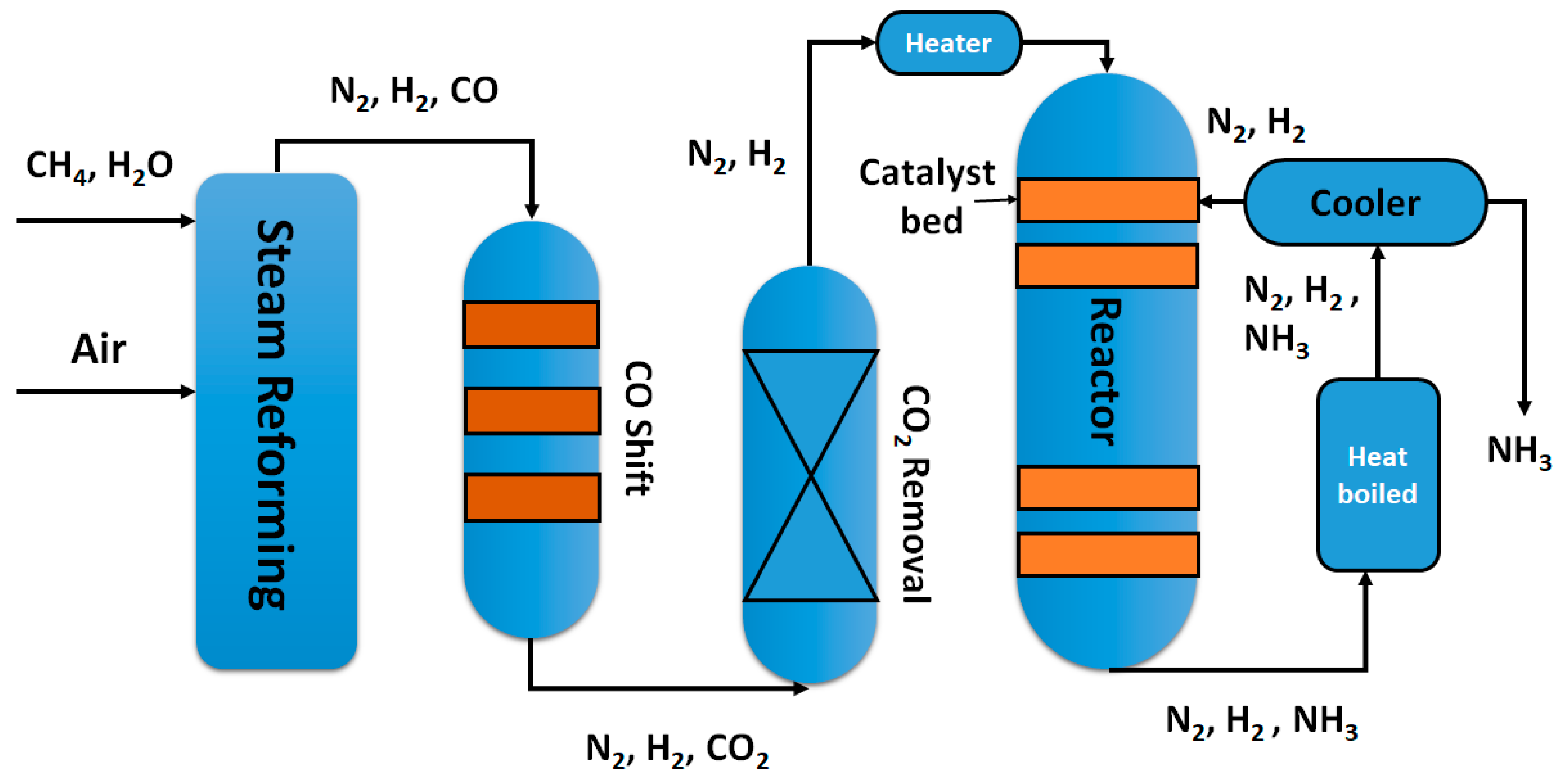 Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
Catalysts Free Full Text Insights Into The Recent Progress And Advanced Materials For Photocatalytic Nitrogen Fixation For Ammonia Nh3 Production Html
 How The Haber Bosch Process Works Feeding The World
How The Haber Bosch Process Works Feeding The World
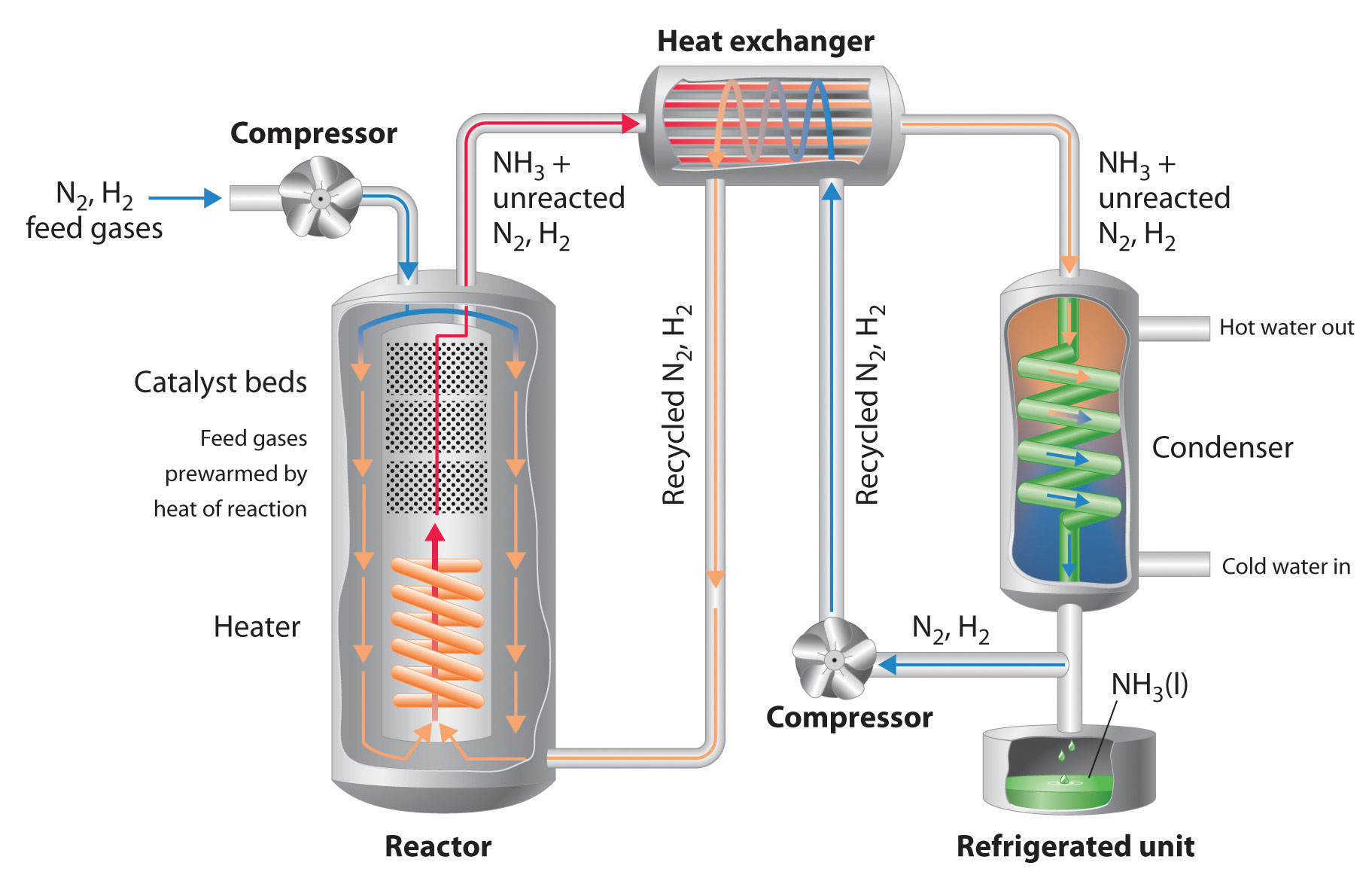 Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts
Chapter 14 6 Controlling The Products Of Reactions Chemistry Libretexts